Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 reduction and salicylic acid decomposition
reduction and salicylic acid decompositionSugita, Tsuyoshi; Mori, Masanobu*; Shimoyama, Iwao
Applied Clay Science, 243, p.107074_1 - 107074_8, 2023/10
Times Cited Count:0 Percentile:0.02(Chemistry, Physical)We have investigated the conversion of biotite, a subgroup of clay minerals, into photocatalysts by heat treatment with CaCl . The reaction products obtained after heat treatment were examined in terms of composition, structure, and photocatalytic activity against Cr
. The reaction products obtained after heat treatment were examined in terms of composition, structure, and photocatalytic activity against Cr and salicylic acid (SA). When mixtures of biotite and CaCl
and salicylic acid (SA). When mixtures of biotite and CaCl were heated at temperatures up to 600
were heated at temperatures up to 600 C, the biotite crystal structure was retained, whereas a phase transformation from biotite to octahedral wadalite crystals occurred upon heating to 700
C, the biotite crystal structure was retained, whereas a phase transformation from biotite to octahedral wadalite crystals occurred upon heating to 700 C. The photocatalytic reduction rate of Cr
C. The photocatalytic reduction rate of Cr per unit surface area and the photocatalytic degradation efficiency of SA increased significantly with increasing treatment temperature. Even the samples that retained the biotite structure after heat treatment displayed some photocatalytic activity, suggesting that this method may also be suitable for preparing photocatalysts from other common natural materials.
per unit surface area and the photocatalytic degradation efficiency of SA increased significantly with increasing treatment temperature. Even the samples that retained the biotite structure after heat treatment displayed some photocatalytic activity, suggesting that this method may also be suitable for preparing photocatalysts from other common natural materials.
 - and K-montmorillonite
- and K-montmorilloniteKawakita, Ryohei; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Kikuchi, Ryosuke*; Otake, Tsubasa*; Sato, Tsutomu*
Applied Clay Science, 231, p.106722_1 - 106722_7, 2023/01
Times Cited Count:1 Percentile:21.06(Chemistry, Physical)Kobayashi, Keita; Yamaguchi, Akiko; Okumura, Masahiko
Applied Clay Science, 228, p.106596_1 - 106596_11, 2022/10
Times Cited Count:6 Percentile:77.65(Chemistry, Physical)no abstracts in English
 Cs
Cs , and
, and 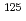 I
I in compacted Ca-montmorillonite; Experimental and modeling approaches
in compacted Ca-montmorillonite; Experimental and modeling approachesFukatsu, Yuta; Yotsuji, Kenji*; Okubo, Takahiro*; Tachi, Yukio
Applied Clay Science, 211, p.106176_1 - 106176_10, 2021/09
Times Cited Count:10 Percentile:75.92(Chemistry, Physical)Yotsuji, Kenji*; Tachi, Yukio; Sakuma, Hiroshi*; Kawamura, Katsuyuki*
Applied Clay Science, 204, p.106034_1 - 106034_13, 2021/04
Times Cited Count:61 Percentile:99.73(Chemistry, Physical)Sugiura, Yuki; Ishidera, Takamitsu; Tachi, Yukio
Applied Clay Science, 200, p.105910_1 - 105910_10, 2021/01
Times Cited Count:6 Percentile:51.59(Chemistry, Physical)Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 195, p.105741_1 - 105741_11, 2020/09
Times Cited Count:2 Percentile:10.08(Chemistry, Physical)Safety functions for the clay buffer in a repository for high-level radioactive waste (HLW) are fulfilled if the presence of montmorillonite with high swelling capacity and low permeability is maintained in the long-term. The transformation of montmorillonite to the non-swelling mineral likely illite is addressed in most safety assessments by using simple semi-empirical kinetic models, but this approach contrasts with more complex reactive-transport simulations. In the present study, reactive-transport simulations are compared with simple semi-empirical kinetic models. Results suggest that reactive-transport simulations err on the side of conservatism, but may produce unrealistic estimates of illitization. This comparison demonstrates that reactive-transport models may be carefully applied to simulate the long-term evolution of near field environment for HLW disposal.

 on Ni-Zn layered hydroxide salt and its application to removal of ReO
on Ni-Zn layered hydroxide salt and its application to removal of ReO
 as a surrogate of TcO
as a surrogate of TcO

Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Onuki, Toshihiko; Kaplan, D. I.*; Grambow, B.
Applied Clay Science, 182, p.105282_1 - 105282_8, 2019/12
Times Cited Count:16 Percentile:67.61(Chemistry, Physical)In this study, Ni-Zn layered hydroxide salt (LHS) was used for adsorption experiments of ReO
 , as a surrogate of TcO
, as a surrogate of TcO
 , in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86
, in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86  10
10 eq/g) by fitting adsorption isotherm of ReO
eq/g) by fitting adsorption isotherm of ReO
 to Langmuir plot. The adsorption of ReO
to Langmuir plot. The adsorption of ReO
 at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl
at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl , NO
, NO
 and SO
and SO
 in solution. EXAFS analysis indicated that ReO
in solution. EXAFS analysis indicated that ReO
 was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
 than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
 by reductive precipitation.
by reductive precipitation.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 179, p.105146_1 - 105146_10, 2019/10
Times Cited Count:13 Percentile:62.41(Chemistry, Physical)Natural systems evidence for the effects of temperature and the activity of aqueous silica upon montmorillonite stability was evaluated. Thermodynamic modeling using three different TDBs shows that stability fields for montmorillonite exist from 0 to 140 C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60
C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60 C to that for illite + quartz at higher temperatures. However, even over very long timescales (
C to that for illite + quartz at higher temperatures. However, even over very long timescales (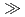 1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80
1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80 C.
C.
Takahashi, Hiroaki*; Tachi, Yukio
Applied Clay Science, 168, p.211 - 222, 2019/02
Times Cited Count:9 Percentile:47.52(Chemistry, Physical)Microstructural and mass transport properties of compacted Na- and Cs-montmorillonites with different swelling properties were investigated by combining 3D microstructure analysis using nanofocus X-ray CT and diffusion measurement of HDO. The X-ray CT observations indicated that macropores in the dry state of compacted Na-montmorillonite are filled with gel phases, and the grain sizes of clay particles shifted toward smaller values through the saturation and swelling processes. By contrast, no gel phase and no decrease in the grain and pore volumes were observed for saturated Cs-montmorillonite. The geometrical factors of the macropores including tortuosity and geometric constrictivity of saturated Cs-montmorillonite determined by the X-ray CT was consistent with the corresponding values derived in the HDO diffusion test. In the case of Na-montmorillonite, the larger differences between the geometric factors evaluated by the X-ray CT and the diffusion tests can be explained by the electrostatic constrictivity factor and the additional geometrical factors in gel phase and interlayer that are smaller than the detection limit of the X-ray CT.
Fukushima, Yoshiaki*; Yamada, Takeshi*; Tamura, Kenji*; Shibata, Kaoru
Applied Clay Science, 155, p.15 - 19, 2018/04
Times Cited Count:5 Percentile:20.86(Chemistry, Physical)Dynamics of a fluoromica (ME100) cation exchanged for dioctadecyl dimethyl ammonium ion (DODA )/ polypropylene composite was analyzed by quasi elastic neutron scattering (QENS), besides XRD and DSC. The QENS spectra for the DODA-ME100 at low Q=2.75 nm
)/ polypropylene composite was analyzed by quasi elastic neutron scattering (QENS), besides XRD and DSC. The QENS spectra for the DODA-ME100 at low Q=2.75 nm were not changed even at temperature higher than 445 K, the melting point of DODA
were not changed even at temperature higher than 445 K, the melting point of DODA . The results suggested the long range (
. The results suggested the long range ( 2 nm) molecular motions in interlayer space are restricted due to the rigid silicate layers and the strong electrostatic interaction between DODA
2 nm) molecular motions in interlayer space are restricted due to the rigid silicate layers and the strong electrostatic interaction between DODA and the ME100. Elastic intensity scan results suggested that a little amount of motion of the polymer chains in the composite was also restricted in the molten state at 445 K. The QENS is expected to be one of the useful tools for studying the composite materials.
and the ME100. Elastic intensity scan results suggested that a little amount of motion of the polymer chains in the composite was also restricted in the molten state at 445 K. The QENS is expected to be one of the useful tools for studying the composite materials.
Okubo, Takahiro*; Ibaraki, Moe*; Tachi, Yukio; Iwadate, Yasuhiko*
Applied Clay Science, 123, p.148 - 155, 2016/04
Times Cited Count:29 Percentile:74.44(Chemistry, Physical)The pore distribution of water-saturated compacted clay (Na-montmorillonite at 0.8 and 1.4 g/cm saturated by three salt concentrations) was evaluated using
saturated by three salt concentrations) was evaluated using  H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature
H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature  H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with
H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with  H NMR relaxometry within 10%. Correlation experiments between longitudinal (
H NMR relaxometry within 10%. Correlation experiments between longitudinal (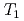 ) and transverse relation times (
) and transverse relation times (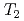 ) at -10
) at -10 C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm
C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm .
.
 on biogenic birnessite
on biogenic birnessiteSasaki, Keiko*; Yu, Q.; Momoki, Taichi*; Kaseyama, Takuya*
Applied Clay Science, 101, p.23 - 29, 2014/11
Times Cited Count:12 Percentile:38.22(Chemistry, Physical) C
COda, Chie; Walker, C.; Chino, Daisuke*; Ichige, Satoru; Honda, Akira; Sato, Tsutomu*; Yoneda, Tetsuro*
Applied Clay Science, 93-94, p.62 - 71, 2014/05
Times Cited Count:7 Percentile:24.59(Chemistry, Physical)Na-montmorillonite dissolution in a 0.3M NaOH solution has been investigated at pH12 and 70 C. The flow-through dissolution experiments were conducted in a dispersed system with varying concentrations of Si and Al to derive a Na-montmorillonite dissolution rate, as a non-linear function of the Gibbs free energy of reaction, dGr. This rate equation was used to simulate the batch-type Na-montmorillonite reaction experiments conducted in a coagulated system. The model simulation of the batch-type experiment adopting the empirical rate equations of Na-montmorillonite dissolution and secondary mineral analcime precipitation were able to reproduce the measured changes in the amount of dissolved Na-montmorillonite and concentrations of Si and Al in solution. The results showed that the empirical rate equation of Na-montmorillonite dissolution determined in the dispersed system was applicable to the coagulated system over a higher dGr range and that the concentrations of Si and Al in the batch experiment were controlled by the precipitation of analcime.
C. The flow-through dissolution experiments were conducted in a dispersed system with varying concentrations of Si and Al to derive a Na-montmorillonite dissolution rate, as a non-linear function of the Gibbs free energy of reaction, dGr. This rate equation was used to simulate the batch-type Na-montmorillonite reaction experiments conducted in a coagulated system. The model simulation of the batch-type experiment adopting the empirical rate equations of Na-montmorillonite dissolution and secondary mineral analcime precipitation were able to reproduce the measured changes in the amount of dissolved Na-montmorillonite and concentrations of Si and Al in solution. The results showed that the empirical rate equation of Na-montmorillonite dissolution determined in the dispersed system was applicable to the coagulated system over a higher dGr range and that the concentrations of Si and Al in the batch experiment were controlled by the precipitation of analcime.
Iijima, Kazuki; Tomura, Tsutomu*; Shoji, Yoshiyuki*
Applied Clay Science, 49(3), p.262 - 268, 2010/06
Times Cited Count:39 Percentile:71.76(Chemistry, Physical)Sorption and desorption behavior of Cs onto montmorillonite colloids was investigated. The ion exchange and surface complexation model can successfully reproduce the sorption and desorption behavior of Cs. Conditioning montmorillonite in higher Cs concentration than 0.005M causes decrease of interlayer distance which may lead to fixation of Cs in the interlayer. Sorption reversibility of Cs on montmorillonite colloids can be expected in this study due to lower Cs concentration than 0.0001M.
Aoki, Kazuhiro; Sugita, Yutaka; Chijimatsu, Masakazu*; Tazaki, Kazue*
Applied Clay Science, 47(1-2), p.147 - 154, 2010/01
Times Cited Count:6 Percentile:21.64(Chemistry, Physical)Microbial activity has been investigated for the bentonite buffer and surrounding host rock (granodiorite) at Kamaishi Mine in Iwate. For the host rock, total number of bacteria and viable microorganisms were enumerated for deep groundwater in granodiorite. Presence of sulphate-reducing bacteria and denitrifying bacteria were also confirmed. The coupled thermo-hydro-mechanical (T-H-M) experiments named" engineered barrier experiments" were carried out to examine the in situ performance of buffer material. At the end of the heating and cooling phases, bentonite samples were taken for microbial analysis to determine if the naturally present microbial population in the buffer material survived the conditions in a simulated vault environment. The results confirmed the existence of heterotrophs, which disappeared in bentonite samples with low water content. These results suggest that microbial activity is severely limited near waste container in the vault for some time after disposal, due to desiccation as a result of the heat output of the waste container. Such knowledge will be useful in assessing the potential effects of microbial activity on deep geological disposal of high level radioactive waste.
Savage, D.*; Benbow, S.*; Watson, C.*; Takase, Hiroyasu*; Ono, Kaori*; Oda, Chie; Honda, Akira
Applied Clay Science, 47(1-2), p.72 - 81, 2010/01
Times Cited Count:37 Percentile:70.43(Chemistry, Physical)Mudstones containing smectite have been altered under mildly alkaline conditions (9  pH
pH  10) at Searles Lake, California over a 3 million-year time period. This natural alteration has been simulated incorporating time-dependent boundary conditions of sedimentation and fluid composition, a Pitzer model for activities of aqueous species, and a coupled hydrogeological model for time-dependent flow in the sediment layers. Kinetic dissolution of detrital smectite under alkaline conditions was described using one of two models based on departure from thermodynamic equilibrium or by an empirical rate dependent upon aqueous Si concentrations. The zonal pattern of smectite dissolution observed at Searles Lake was reproduced reasonably well by the "Cama-TST" model of montmorillonite dissolution. This assessment provides a test of the accuracy and reliability of published data in the application of models of smectite dissolution in the long-term.
10) at Searles Lake, California over a 3 million-year time period. This natural alteration has been simulated incorporating time-dependent boundary conditions of sedimentation and fluid composition, a Pitzer model for activities of aqueous species, and a coupled hydrogeological model for time-dependent flow in the sediment layers. Kinetic dissolution of detrital smectite under alkaline conditions was described using one of two models based on departure from thermodynamic equilibrium or by an empirical rate dependent upon aqueous Si concentrations. The zonal pattern of smectite dissolution observed at Searles Lake was reproduced reasonably well by the "Cama-TST" model of montmorillonite dissolution. This assessment provides a test of the accuracy and reliability of published data in the application of models of smectite dissolution in the long-term.
Sato, Haruo
Applied Clay Science, 29, p.267 - 281, 2005/00
Times Cited Count:26 Percentile:64.44(Chemistry, Physical)The concept of air transport of A Type package containing nuclear fuel materials according to the nuclear disaster countermeasures law, and the experience of a transportation of plutonium solution from France are shown.
Nakayama, Shinichi; Sakamoto, Yoshifumi; Yamaguchi, Tetsuji; Akai, Masanobu; Tanaka, Tadao; Sato, Tsutomu*; Iida, Yoshihisa
Applied Clay Science, 27(1-2), p.53 - 65, 2004/10
Times Cited Count:81 Percentile:89.26(Chemistry, Physical)Alkaline environments induced by cement in radioactive waste repositories are likely to alter montmorillonite, the main constituent of bentonite buffer materials. Over long time periods, the alteration may cause the physical and/or chemical properties of the buffer to deteriorate. For the purpose of acquiring numerical data to quantify the effect of alteration on permeability of bentonite buffer, dissolution rates of montmorillonite and diffusivity of hydroxide ions in compacted sand-bentonite mixture specimens have been measured under highly alkaline, simulated groundwater conditions. The dissolution rate of montmorillonite was given by the linear dependence on time under the employed experimental conditions of pH 13 to 14 and temperatures of 90 to 170 C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50
C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50 C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10
C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10 to 10
to 10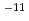 m
m /s.
/s.
Sato, Haruo
Applied Clay Science, 26, p.47 - 55, 2004/00
Times Cited Count:29 Percentile:67.22(Chemistry, Physical)None